API development

As a pharmaceutical innovator, manufacturing a new API may seem both complex and challenging. The development and optimization of a robust, transferable, and scalable process to support long-term manufacturing requires both specialized capacities and expertise.
Axplora can bring its extensive process development experience to your drug substance, from technical and analytical transfer to process optimization and validation. We offer a full range of CDMO services for your APIs, with an emphasis on quality, speed, and flexibility.
Recognized experts in API process development and scale-up
Axplora has been a leading player in the CDMO development and manufacturing area for more than thirty years.
With more than fifty process steps transferred from R&D to manufacturing each year, Axplora is recognized for its process development know-how. Our strong expertise in DoE (Design of Experiments) enables us to increase process robustness, ease manufacturing scale-up, and accelerate timelines to fulfill your needs.

An extensive toolbox of technologies, coupled with our strength in process chemistry, allows our teams to develop and implement innovative new-generation processes, and extend product lifecycles.
“Axplora’s experts have helped us a lot in overcoming technical problems in the process over the last few years.”
Leading pharma company
Our reliable CDMO services for API development
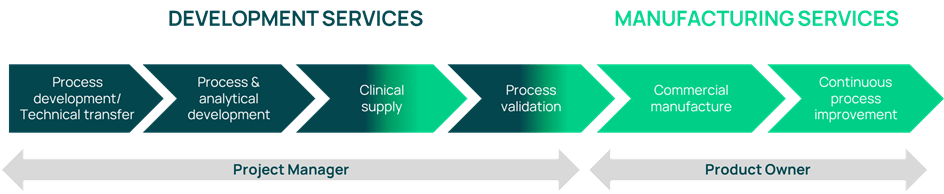
From clinical supply to commercial manufacture, Axplora offers a full range of process development and manufacturing services for APIs.
- Process & analytical development to scale up
- Process & analytical method development
- Process characterization
- Process safety assessments
- Technical transfer
- Validation
- Analytical method validation
- Cleaning method validation
- Equipment validation
- Regulatory support
- Process validation
- Preparation of validation protocols
- Control of SM quality
- Production of validation batches
- Process and quality review
- Preparation of validation report
Axplora can support your drug development lifecycle from clinical to commercial manufacturing.

A dedicated Project Manager is assigned to each project to support you throughout your API product lifecycle and ensure the objectives are met (quality, costs, timelines).
Our facilities for API development
Our global network of ten sites, located in Europe, India, and the U.S., is strongly supported by a team of committed chemistry professionals.
| Sites | Process development | cGMP Pilot | cGMP commercial |
| Chasse-sur-Rhône, FR | X | X | X |
| Chennai, IN | X | X | |
| Gropello Cairoli, IT | X | X | X |
| Le Mans, FR | X | X | X |
| Leverkusen, DE | X | X | X |
| Mourenx, FR | X | X | X |
| Pompey, FR | X | X | X |
| Raubling, DE | X | X | X |
| Vizag, IN | X | X | X |
| Wilmington, US (non-GMP) | X |
Our technologies
Discover why Axplora is a first-choice CDMO partner for your API development and manufacturing:








