API manufacturing

Small molecule API manufacturing is a complex challenge requiring chemistry expertise as well as specialized facilities, regulatory experience & supply chain management.
For more than thirty years, Axplora has offered API (Active Pharmaceutical Ingredient) manufacturing services, from process development to commercial manufacturing in full regulatory compliance, to ensure a robust and secure supply chain for your APIs, while reducing risks and optimizing costs.
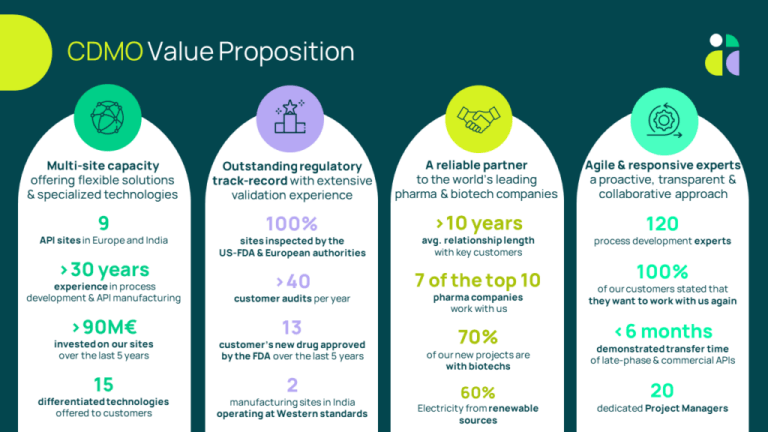
A reliable CDMO partner for API manufacturing
For more than 30 years, Axplora has been a leading CDMO in the API (Active Pharmaceutical Ingredient) arena, capable of providing unique services for drug substance manufacturing from clinical to commercial phases.
- With an experienced team of chemists, Axplora:
- Manufactures more than forty commercial APIs & advanced intermediates per year;
- Has validated more than fifty chemical steps in the last three years;
- Has manufactured 14 customer products that received FDA approval over the last five years
- What makes Axplora a trusted API manufacturing partner?
- Outstanding quality assurance track record
- Multi-site API manufacturing capabilities
- Expertise in specialized technologies
- Broad toolbox of classical chemistry
- Flexible and collaborative approach
- Complex and multi-step synthesis
- Extensive validation experience
- Strength of process chemistry
Did you know?
Axplora is honored to be a recipient of 2023 CDMO Leadership Awards in four categories:

We also achieved Champion status in Expertise and Service:
First-class API manufacturing services
Axplora offers a full range of CDMO services for APIs to pharmaceutical innovators, from kilograms up to hundreds of tons.
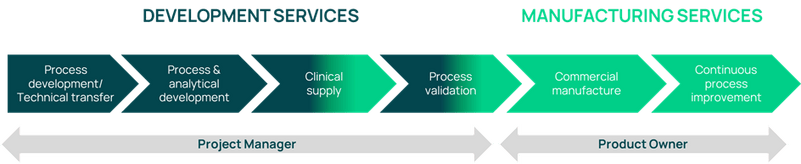
Regardless of the development phase of your API, we offer seamless development and scale-up services to provide robust long-term manufacturing solutions.
To support your drug manufacturing and prepare for your commercial launch, our cGMP services include:
- Process development:
- Process development & scale-up
- Analytical methods development, optimization, and validation
- Process validation
- Technical transfer
- Safety assessment
- Regulatory support
- ICH stability studies
- Manufacturing:
- Kg-lab, pilot, and manufacturing scale
- A wide range of flexible reactor trains from 30L to 12.5m³
- Clean rooms class 100’000
- 9 API manufacturing sites inspected by the FDA & EU authorities
- Clinical & commercial manufacturing
- Continuous improvement
As you work to bring your product to market, we aim at continuously streamlining and optimizing your API production processes to offer cost-efficient solutions.

A dedicated Project Manager is assigned to each project to ensure the objectives are met (quality, costs, timelines), keep your clinical program on track, and support you throughout your API product lifecycle.
Supporting technologies for custom API manufacturing
Manufacturing your API with us also means leveraging advanced technologies such as:
- Low-temperature chemistry
We are also a reliable and experienced manufacturing partner for Palladium cross-coupling reactions such as Buchwald, Cyanation, Heck, Sonogashira & Suzuki, and many other reactions.

Nine API cGMP manufacturing sites
Located in Europe and India, all our nine production sites have broad experience in API manufacturing. The power of Axplora’s network lies in its ability to manage several steps of chemistry through different sites to provide flexible capacity and increase the security of supply.
| Sites | Process development | cGMP Pilot | cGMP commercial |
| Chasse-sur-Rhône, FR | X | X | X |
| Chennai, IN | X | x | |
| Gropello Cairoli, IT | X | X | X |
| Le Mans, FR | X | X | X |
| Leverkusen, DE | X | X | X |
| Mourenx, FR | X | X | X |
| Pompey, FR | X | X | X |
| Raubling, DE | X | X | X |
| Vizag, IN | X | X | X |
Our manufacturing plants offer a combined reactor capacity of over 600m3 with a wide range of equipment trains.
Our facilities enable us to offer a multi-site strategy to leverage our core technologies and best accommodate our customers’ needs. For example, Axplora is highly experienced in combining synthesis preparative chromatography and has access to one of the largest chromatographic purification platforms worldwide.
Discover why Axplora is a first-choice CDMO partner for your API manufacturing:

Our latest investments
Axplora has deployed a long-standing investment strategy of continuously upgrading capacities and broadening our flexibility and productivity to meet our clients’ needs and ultimately, their patients’ needs.
-
- Support growing demand for sustainable chemistry
- Versatile equipment designed for clinical supply of APIs & intermediates to customers
- Announced in July 2023
- Located in Leverkusen, Germany
-
- Debottlenecking of drying and purification capacities, and addition of a cleanroom
- Investment supported by ‘France Relance’
- Announced in December 2021
- Located in Chasse-sur-Rhône, France
-
- Increase flexibility and competitiveness
- Investment supported by ‘France Relance’
- Announced in June 2021
- Located in Mourenx, France
-
- Support growing demand for clinical and commercial supply of ADCs & HPAPIs
- Announced in May 2021
- Located in Le Mans, France






