A new cGMP pilot facility for flow chemistry
Published on July 25, 2023
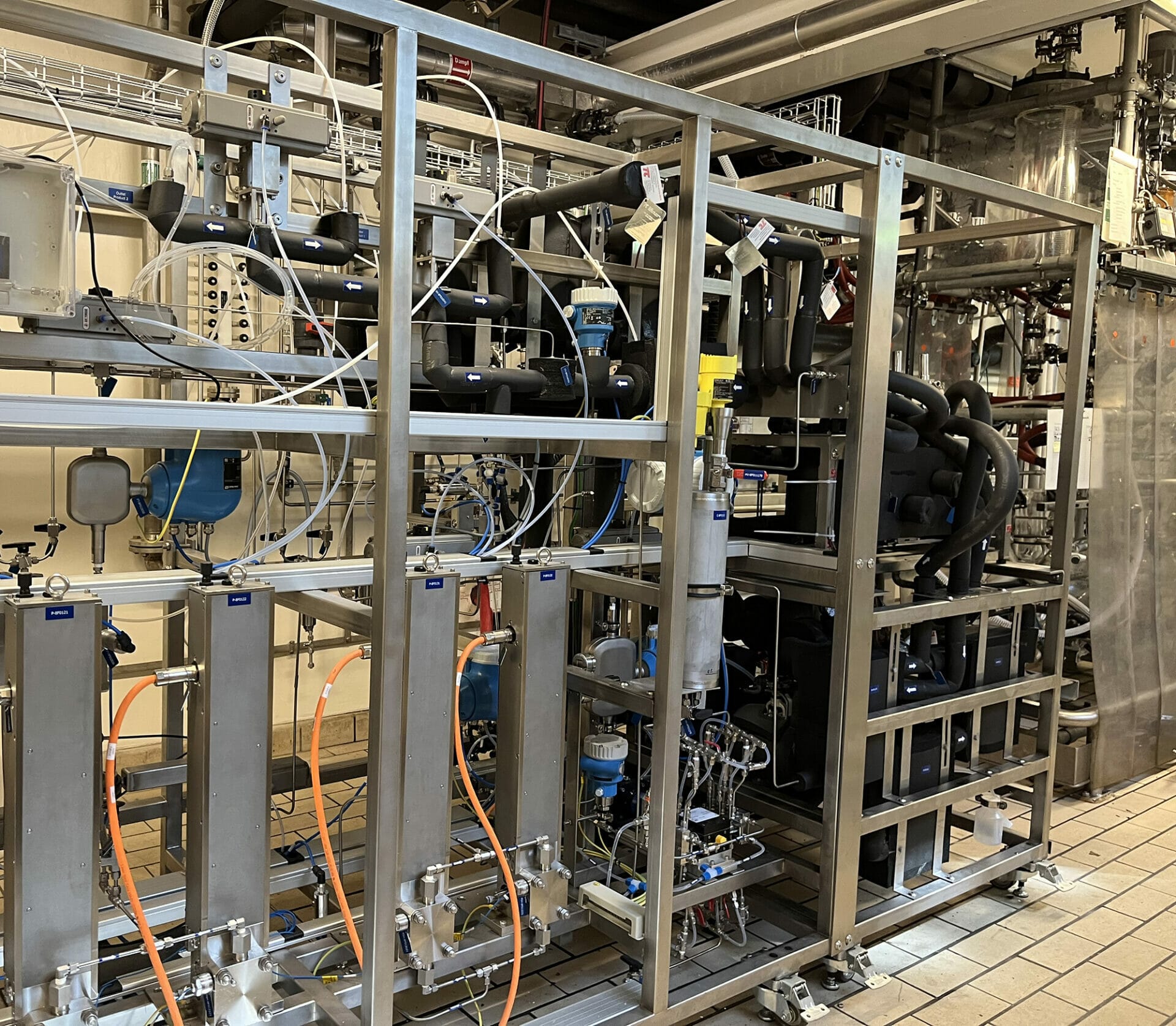
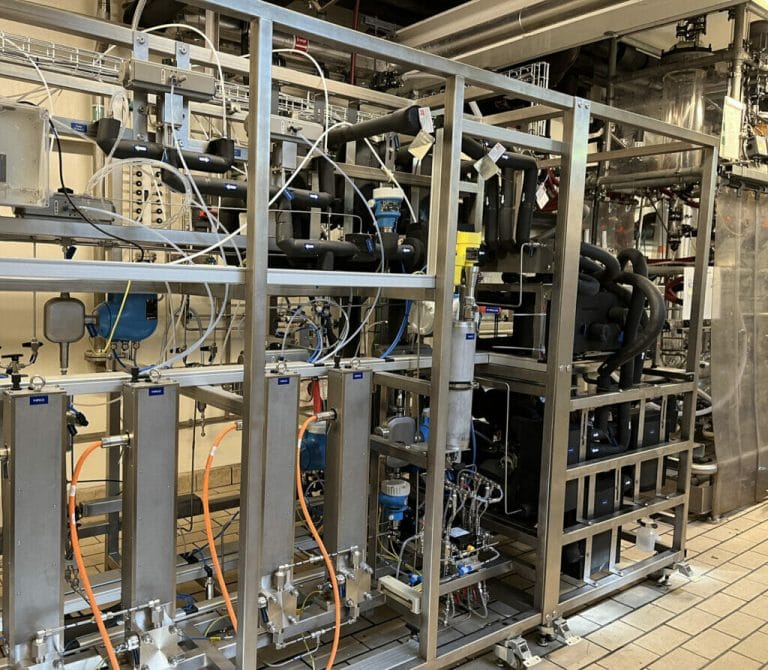
- In response to the growing demand for sustainable chemistry, this €1M+ cGMP pilot flow chemistry unit reinforces Axplora’s capabilities in specialized technologies and consolidates its position as a leading CDMO for continuous processes.
- The versatile equipment is designed for clinical supply of APIs & intermediates to customers, enabling them to cut down costs and time to market to serve their patients.
- Capable of operating at a broad range of temperatures and pressures, this new facility opens access to a wide variety of reactions to support the development of sustainable & cost-effective processes.
Successful installation of a new cGMP pilot flow chemistry unit at Axplora’s Leverkusen site
Raubling, 25 July 2023 – Axplora, a leading partner to Pharma companies and Biotechs for complex APIs, is pleased to announce the successful installation of a new cGMP pilot unit at its Leverkusen site (Germany), continuing its investment in differentiating and specialized technologies.
Underpinned by innovation, Axplora has long been working on developing new technologies, such as flow chemistry, to operate even more efficiently and sustainably. In 2013, Axplora established a dedicated research platform at its Chasse-sur-Rhône site (France), to develop methodologies for efficient process development under continuous flow conditions, using chemical and engineering expertise.
This high flexible equipment allows Axplora to operate at a broad range of temperatures and pressures (-50°C up to 200°C and up to 40 bar), with reactor configurations adapted to the specific requirements of the process & optimize control and productivity of highly demanding reactions.
“Continuous flow technology can improve the robustness, sustainability and safety of many chemical processes. It is an enabling technology that allows the safe generation and use of hazardous intermediates, as well as operation under high temperatures and pressures”,says Dr. David Cantillo, renowned Flow Chemistry Expert and Area Leader at the Research Center Pharmaceutical Engineering GmbH (RCPE) & Assistant Professor at the University of Graz (Austria). “Furthermore, the enhanced heat and mass transfer characteristic of flow reactors improve the outcome of fast and exothermic transformations.”
“In line with our commitment to help our customers deliver the best medicines for their patients, we are thrilled to announce the installation of a flow chemistry pilot unit at our Leverkusen site”, adds Dr. Ester Masllorens, Chief Technology Officer at Axplora. “Continuous processing is a key enabling technology for the future of pharmaceutical manufacturing, strongly supported by regulatory bodies such as the FDA. This technology has high potential to enhance sustainability, improve control and quality as well as reduce costs and time to market. My warm thanks to our team involved in making it possible.”
“Hazardous chemistry is our core competence at Leverkusen”,ends Dr. Oliver Plietzsch, Head of Research & Development at Axplora’s Leverkusen site. “We are excited to implement flow chemistry at pilot scale as it enhances our possibilities to develop safe, sustainable and efficient processes for our customers.”
About Axplora
Axplora is the preferred API manufacturing partner to the world’s leading patient-centric pharmaceutical and biotechnology companies, delivering top quality active ingredients on time and at scale, to the highest industry standards. Axplora offers CDMO solutions to innovators for their small molecule and biomolecular active pharmaceutical ingredients, as well as products that address respiratory, inflammatory and liver diseases. Axplora is dedicated to serving pharmaceutical companies make critical medicines faster, reliably, and sustainably benefitting patients worldwide. Explore more: axplora.com
Press contact
Thomas Goldberg
+33 (0)4 37 28 20 52 | +33 (0)6 03 78 55 63

